Table of Contents
PREFRONTAL CORTEX
Introduction
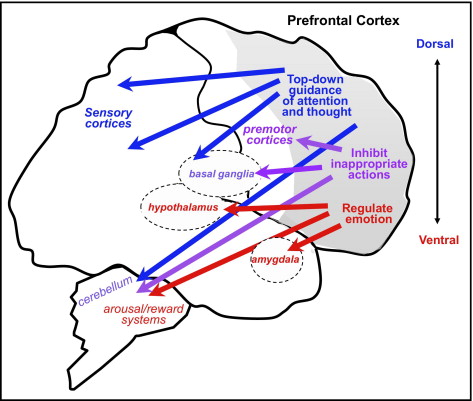
The prefrontal cortex (PFC) governs the executive control of information processing and behavioral expression, including the ability to selectively attend and maintain information, inhibit irrelevant stimuli and impulses, and evaluate and select the appropriate response . This cognitive and behavioral control facilitates successful achievement of complex goal-directed behaviors. Some evidence suggests that all regions of the PFC (dorsal, ventral, lateral, medial) have the capacity to perform the same type of executive control functions (i.e. evaluate, maintain, inhibit, or select) [(Duncan and Owen 2000)]. However, other evidence indicates that particular regions of the PFC are biased toward specific functions and information domains (Shimamura 2000,Muller et al. 2002).
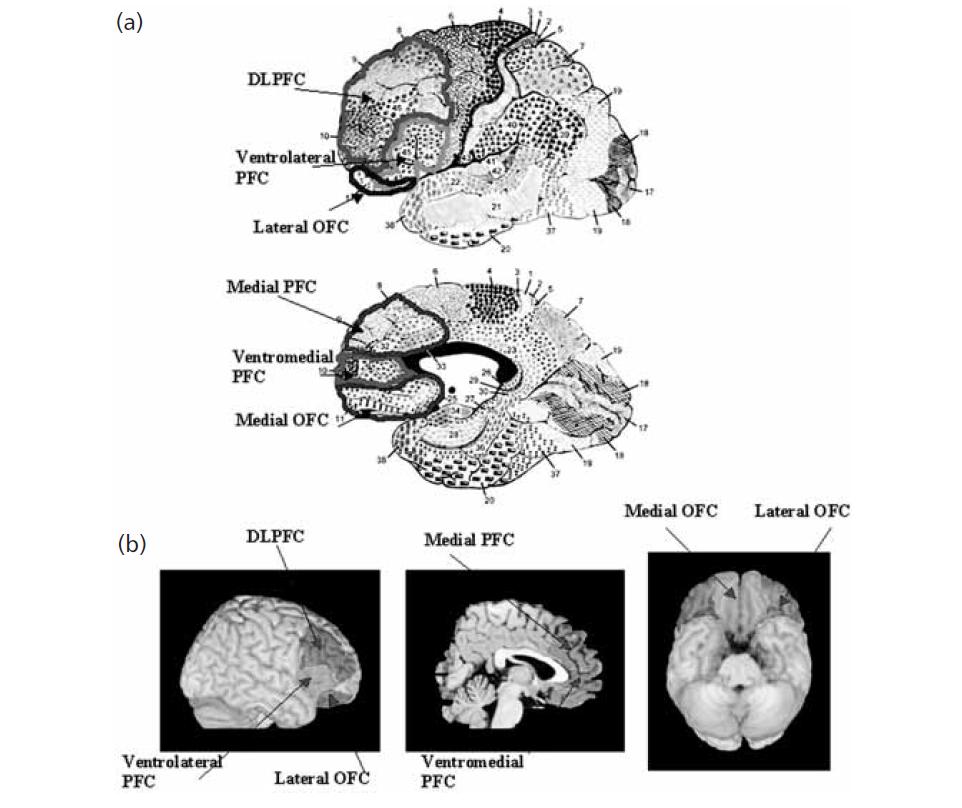
OFC
Connections
- receives inputs from every sensory modality
- has immense reciprocal connections with subcortical structures, such as
- the amygdala, thalamus, and periaquaductal gray area
- Dopamine projections from the nucleus accumbens and ventral tegmental area also project significantly to the orbitofrontal cortex
- has direct projections to the primary and secondary sensory cortices
- closely connected to the dorsolateral prefrontal cortex.
- reciprocal projections to granular, dysgranular, and agranular insular cortex, parahippocampal regions, and the hippocampus, particularly CA1 regions in a rostral-to caudal gradient.
- The orbitofrontal cortex additionally shares extensive reciprocal connections with the amygdala, and direct and indirect connections to the hypothalamus.
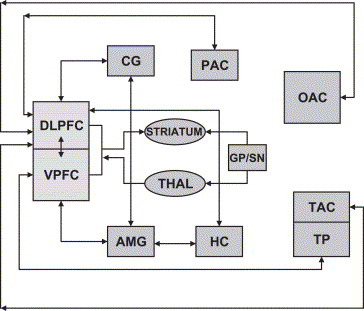
- Additional subcortical projections are shared between the striatum, particularly ventral reward-related areas[9],[10]. Connectivity with thalamic and periaqueductal grey areas further suggests a role for the orbitofrontal cortex in both inhibitory and excitatory regulation of autonomic function[6]. Parallel processing loops in connectivity between cortico-striatal networks seem to be involved in the processing of goal-directed and habitual action, whereas cortico-limbic connectivity seems to be of prime importance for action selection, implicating the basolateral amygdala, and the integration of information into behavioral output[11]
- The OFC projects and is neuronanatomicaly connected (via the mediodorsal nucleus of the thalamus) to the nucleus acumbens - which is associated with the reinforcing effects of drug administration [26][27][28]. The nucleus acumbens projects back to the OFC[29] , as do dopamine cells in the ventral tegmental area (VTA) [30] - the latter being associated with the positive, reinforcing effects of drugs. Limbic regions including the amygdala, hippocampus and cingulated gyrus also project to the OFC via direct and indirect pathways[27][31] and it would appear that the OFC is not only the target for reinforcing drug effects but also serves to integrate information from the limbic system, modulating the response of the limbic areas to drugs of abuse (and their rewarding effects)[16].
Function
- dysregulated OFC connectivity/circuitry center problems : decision-making, emotion regulation, and reward expectation
- problems with decision-making is drug addiction/substance dependence, which can result from disruption of the striato-thalamo-orbitofrontal circuit[13][15][16]. Attention Deficit Hyperactivity Disorder (ADHD) has also been implicated in dysfunction of neural reward circuitry controlling motivation, reward, and impulsivity, including OFC systems[14]. Other disorders of executive functioning and impulse control may be affected by OFC circuitry dysregulation, such as Obsessive-Compulsive Disorder and trichotillomania[17][18][19]
- reward value, the expected reward value, and even the subjective pleasantness of foods and other reinforcers are represented in the OFC
- can modulate the strength of the neural signal coming from sensory cortex,regulating the influence of that sensory signal on the rest of the brain, and, ultimately, on behavior.
- suppresses neural activity related to aversive or painful sensations
- OFC lesion neural response not habituate.
- normally inhibits aversive sensations very early in sensory processing by regulating neural activity in posterior sensory association areas.
- OFC lesion patients displayed more surprise behavior and reported more fear
than the normal controls in response to the noise.
- calculates or represents the emotions that individuals would experience if they pursued each alternative.These emotions then bias the decisions that individuals reach.
- enables individuals to anticipate whether some course of action will evoke positive or negative emotions, both immediately and in the future.
- moral behavior. That is, when the orbitofrontal cortex is activated, individuals choose the courses of action that tend to be rewarded, rather than punished, by other people.
- improve future predictions - adapt to unexpected outcomes.
- facilitates empathy.
- OFC neurons are activated by rewards and cues that predict rewards in a way that reflects their incentive value, typically independent of the predictive cue or associated response
- OFC represents bad outcomes as well as good ones
- OFC plays a critical role in evoking the original learning and integrating it with the new value of the outcome to guide responding.
- Damage to OFC and medial PFC is associated with increased risk for the display of reactive
aggression especially when the lesion occurs in childhood
MOFC
- learning, and memory of the reward value of reinforcers
- When people allowed themselves to feel
the full negative impact of negative scenes, medial OFC, insula, and amygdala, were active
LOFC
- evaluation of punishers, which may lead to a change in ongoing behaviour
- LOFC (bilaterally) + and DLPFC (bilaterally) + during
anticipation of pain predict less pain related activity in the thalamus, insula and dorsal ACC. In addition, both the OFC and DLPFC activity correlated with self-report of decreased pain. These findings add a temporal component and suggest that the OFC can inhibit pain sensation by increasing activity in the anticipation of a painful stimulus.
- facilitates selective attention by controlling the interference of
irrelevant emotional information in the environment on spatial attention.
- The faces had either a neutral or fearful expression. The
results showed that the lateral OFC was engaged when fearful faces had to be ignored in order to make a judgement about the houses. However, the lateral OFC was not active when ignoring neutral faces.
- enhanced attention by suppressing the neural activity
associated with the representation of the interfering stimulus
- L-lateral OFC/VLPFC as well as L-DLPFC were both involved in ignoring the fearful face.
+ in these regions in anticipation of a “to be ignored” fearful stimulus, suggesting that these subjects are employing VLPFC activity to help them suppress the interfering influence of the threat related stimulus when the emotional information is not relevant to the task. However, people high in anxiety did not recruit these regions to help them ignore the fearful stimuli, suggesting a failure in top down regulatory control in anxiety
- multiple PFC regions, including the lateral OFC/VLPFC, control emotional experience and expression
- depressed individuals had more R-lateral OFC/VLPFC activity when
ignoring sad words (and attending to happy words) as compared to ignoring happy (and attending to sad words).
- inverse correlation with the L-lateral OFC and the
influence of mood priming on betting. This shows that the lateral OFC regulated the influence of emotion on decision-making
- L-lateral OFC was also involved when subjects were instructed to use
the emotional information as part of their betting strategy. This suggests that the lateral OFC may be involved in inhibiting emotional information when it is irrelevant and integrating it when it is relevant to the decision.
- more right lateral OFC activity in response to angry
expressions as compared to other facial expressions
- in addition to providing the neural mechanism of inhibition, the
lateral OFC may also register and implement signals indicating the need to inhibit, such as an angry or embarrassed facial expression
VLOFC
- effect of placebo - increased lateral OFC/VLPFC activity is related to - pain
- dorsal ACC(-( Between pre- and post-testing placebo
- R-VLPFC mediates a conscious, cognitive evaluation and expectation associated with placebo T
- facilitates working memory by inhibiting proactive interference
Proactive interference occurs when a recent but irrelevant memory interferes with current recognition or recall.
- Greater left VLPFC activity is associated with a
correct response on trials containing a familiar (“lure”) item. In other words, greater VLPFC activity is associated with resistance to proactive interference .
- VLPFC help maintain goal oriented focus by suppressing the influence of interfering
information
- when subjects reappraised
negative scenes so that their interpretation decreased their negative feelings, the VLPFC (inferior frontal gyrus BA 44, 10, 46) and the DLPFC were active . In addition, lateral OFC and VLPFC (BA 44,46) activity during reappraisal was inversely correlated with activation in the amygdala.
- VLPFC and DLPFC activity have been observed in emotion regulation,
there is evidence that lateral OFC is particularly involved in strategies employed to decrease negative affect.
- Decreasing distress by reappraisal employs VLPFC- and lateral OFC-mediated
top-down regulation
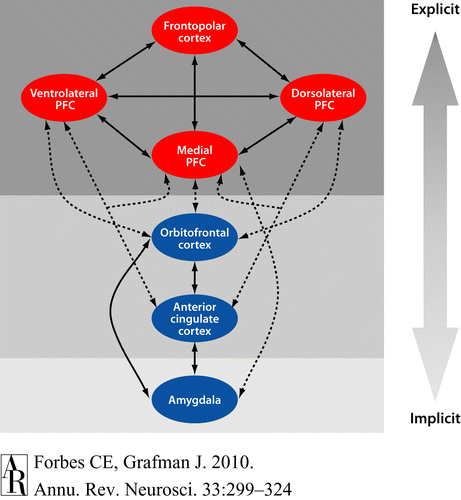
 a | The proposed model. b | A schematic of the functional connectivity of one hemisphere of the orbitofrontal cortex28, 109. Information in the figure flows from bottom to top. Sensory information arrives from the periphery in the primary sensory cortices, where the stimulus identity is decoded into stable cortical representations. This information is then conveyed to brain structures in the posterior parts of the orbitofrontal cortex for further multimodal integration. The reward value of the reinforcer is assigned in more anterior parts of the orbitofrontal cortex, and from here it can be used to influence subsequent behaviour (in lateral parts of the anterior orbitofrontal cortex with connections to the anterior cingulate cortex), stored for learning and memory (in medial parts of the anterior orbitofrontal cortex) and made available for subjective hedonic experience (in the mid-anterior orbitofrontal cortex). The reward value and subjective hedonic experience can be modulated by hunger and other internal states. Important reciprocal information flows between the various regions of the orbitofrontal cortex and other brain regions.
a | The proposed model. b | A schematic of the functional connectivity of one hemisphere of the orbitofrontal cortex28, 109. Information in the figure flows from bottom to top. Sensory information arrives from the periphery in the primary sensory cortices, where the stimulus identity is decoded into stable cortical representations. This information is then conveyed to brain structures in the posterior parts of the orbitofrontal cortex for further multimodal integration. The reward value of the reinforcer is assigned in more anterior parts of the orbitofrontal cortex, and from here it can be used to influence subsequent behaviour (in lateral parts of the anterior orbitofrontal cortex with connections to the anterior cingulate cortex), stored for learning and memory (in medial parts of the anterior orbitofrontal cortex) and made available for subjective hedonic experience (in the mid-anterior orbitofrontal cortex). The reward value and subjective hedonic experience can be modulated by hunger and other internal states. Important reciprocal information flows between the various regions of the orbitofrontal cortex and other brain regions.
 b | The sulcal variability of the human orbitofrontal cortex is shown with ventral views of the three main patterns of gyri and sulci, taken from the left hemisphere.
b | The sulcal variability of the human orbitofrontal cortex is shown with ventral views of the three main patterns of gyri and sulci, taken from the left hemisphere.
TESTS
Visual discrimination test * “reversal learning” * “extinction”
Iowa gambling task
Faux pas test
- posterior-anterior distinction was found with more complex or abstract reinforcers (such as monetary gain and loss) being represented more anteriorly in the orbitofrontal cortex than less-complex reinforcers such as taste
- medio-lateral gradient for representation of positive and negative stimuli, respectively, and an anterio-posterior gradient for complex (higher-order/polymodal) and simple (lower-order/unimodal) stimuli,However, some studies have found medial OFC activity to negative stimuli or lateral OFC activity to positive stimuli . Key issues in understanding these discrepancies include sensory modalities of presented stimuli, stimulus characteristics that may confound valence differences, and individual subject differences, such as menstrual cycle phase in female subjects.
- For example, the OFC (BA 11) and VLPFC (BA 47) are
active when subjects try to decrease their erotic feelings to erotic films as well as when they decrease their feelings of sadness to sad films
- Additionally, greater activity in the right OFC and right DLPFC was associated with
more intense feelings of sadness during the suppression condition, indicating that more intense feelings of sadness required more OFC mediated inhibitory strength to suppress.
- In a direct comparison of using reappraisal to increase negative
affect (i.e. up-regulation) as compared to decrease negative affect (i.e. down-regulation), found that the lateral OFC was more active when using reappraisal to decrease as opposed to increase negative affect, and the DLPFC was more active when using reappraisal to increase as opposed to decrease negative affect. This modulation of amygdala activity occurred within 2 seconds of employing the reappraisal strategy
- DLPFC is involved in broad regulatory control of experience,
especially in bringing emotional representations on line, maintaining them, and strengthening them, whereas the lateral OFC is involved in the inhibition of these cortically represented feelings and images
- Inhibitory control in attitude regulation and memory
Goel and Dolan (2003) had people identify the validity of a set of logical arguments. Some of the arguments were congruent with common beliefs about the world and some were equally valid arguments but contradictory to common world beliefs. Using fMRI they found that right OFC/VLPFC (BA 47, 45) was more active for correctly inhibiting belief bias as compared to error trials in which the belief bias led to faulty reasoning and the wrong conclusion. The VMPFC showed the opposite pattern. It was less active during the correct inhibition of belief bias but more active when the a priori beliefs led to incorrect reasoning (Goel and Dolan 2003). These findings illustrate that right OFC/VLPFC activity was related to resistance to belief bias and suggests that this region may have facilitated this resistance by inhibiting the influence of the a priori belief.
- They found that activity in the right lateral OFC was significantly correlated
with the amount that people felt that they had to control or suppress their initial response to the concept. Interestingly, post-scanning questionnaires revealed that topics which required the suppression of automatic responses were often those topics that had both positive and negative qualities. Therefore these topics required more evaluation before a judgement could be made (Cunningham et al. 2003, 2004), suggesting that, perhaps, the process of evaluation suppresses the influence of emotional response on judgement.
- is not clear if there are distinct functions of the right versus left lateral
OFC/VLPFC during inhibition.Many inhibitory tasks show bilateral activations during inhibition . Though it is tempting to claim that more emotional tasks, such as emotion regulation, may employ right hemisphere systems , and more cognitive tasks, such as the inhibition of proactive interference, use left hemisphere systems, this does not seem to be the case, since some cognitive tasks, such as modulation of belief reason, and some emotional tasks, such as inhibiting a fearful face, show only left hemisphere involvement
There are several issues regarding the inhibitory mechanism that need to be resolved in future research. First, it is not clear if there is a distinction between the inhibitory function of the lateral OFC (BA 11, 12, 47) and the VLPFC, particularly the more superior portions of BA 44, 45, in the frontal operculum. Many neuroimaging studies show inhibitory activity that extends across both regions
- For example, the severity of depression correlates inversely with physiological activity in parts of the posterior lateral and medial OFC, consistent with evidence that dysfunction of the OFC associated with cerebrovascular lesions increases the vulnerability for developing the major depressive syndrome.
- There is abundant evidence for OFC involvement in motivational operations (8). Rapid stimulus-reinforcement association learning is implemented in the OFC (8), and the OFC is important for the alteration of stimulus–reward associations (9). The OFC appears to respond to the pleasantness of some stimuli (10, 11) as well as to the unpleasantness of other stimuli (8, 12–15).
- anteromedial OFC +depressed versus the remitted phases of major depressive disorder to + correlated with the severity of depression.
- Effective antidepressant treatment act(-) . some orbitofrontal and medial prefrontal cortex regions function to inhibit, while others function to enhance, emotional expression.
OFC http://www.psych-it.com.au/Psychlopedia/admin/RTE/my_documents/my_pictures/Orbitofrontal.gif
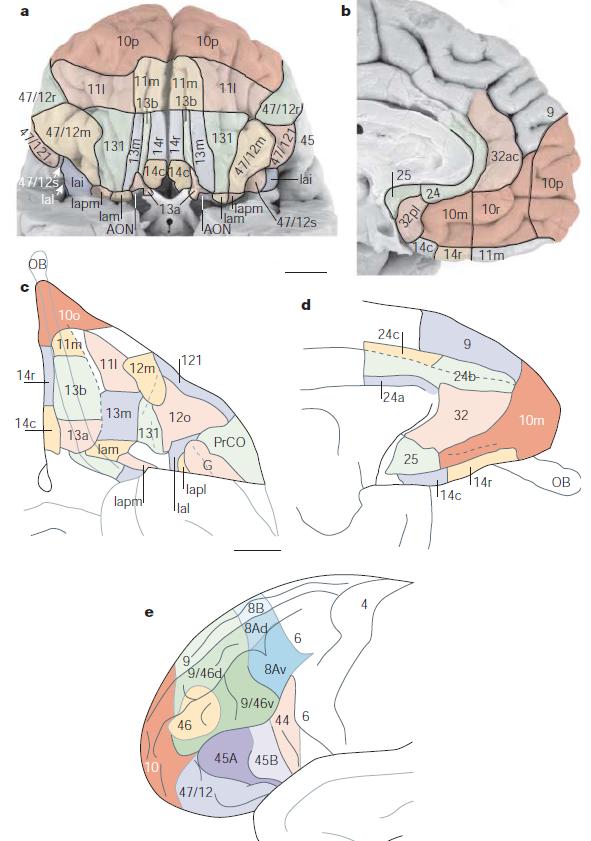
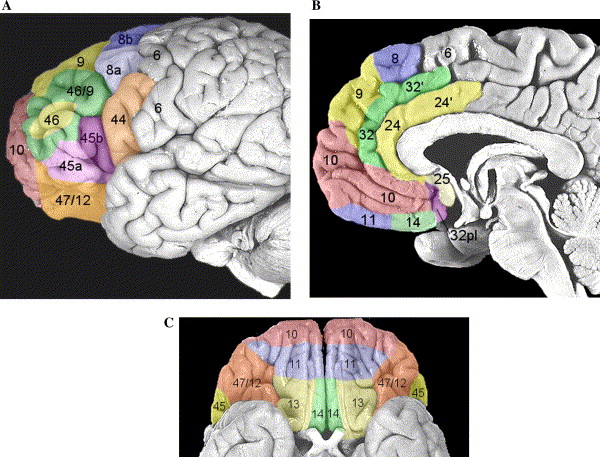
More precisely, the orbitofrontal cortex entails Brodmann areas 10, 11, and 47 (Kringelbach, 2005).
Frontopolar cortex
- anterior prefrontal, rostral prefrontal cortex and frontopolar prefrontal cortex are used to refer to the area in the most anterior part of the frontal cortex that approximates to or principally covers BA10.
- implicated in exploratory decision making
- it is the only prefrontal region that is predominantly (and possibly exclusively) interconnected with supramodal cortex in the PFC, anterior temporal cortex and cingulate cortex.“[8] It has been proposed that due to this connectivity that it can “play a major role in the highest level of integration of information coming from visual, auditory, and somatic sensory systems to achieve amodal, abstract, conceptual interpretation of the environment .. and may be the anatomical basis for the suggested role of the rostral prefrontal cortex in influencing abstract information processing and the integration of the outcomes of multiple cognitive operations
Subareas BA 10 is divided into three sub-areas, 10p, 10m and 10r. 10p occupies the frontal pole while the other two cover the ventromedial part of the prefrontal cortex
http://rstb.royalsocietypublishing.org/content/362/1481/887.full
- also termed ‘rostral prefrontal cortex (PFC)’, ‘anterior PFC’ or ‘frontopolar cortex’
- prospective memory (PM) problems: ‘…it was as if he forgot to remember short- and intermediate-term goals…’
- episodic memory tasks were associated with lateral area 10 activations.
- mentalizing tasks tending to provoke activations within caudal medial aspects of BA 10 , but paradigms that required the coordination of two or more activities (dual task, PM, etc.) were associated with very rostral activations within area 10
- medial rostral PFC are involved in (i) focusing attention on perceptual versus self-generated information or (ii) mentalizing.
- rostral prefrontal cortex (PFC; approximating area 10) supports a cognitive system that facilitates either stimulus-oriented (SO) or stimulus-independent (SI) attending.
- medial area 10 support SO attending is the behaviour required to concentrate on current sensory input, whereas lateral area 10 support processes SI attending is the mental processing that accompanies self-generated or self-maintained thought.
- In particular, there is a functional dissociation between the anterior medial area 10, which supports processes required for SO attending, and the caudal medial area 10, which supports processes relating to mentalizing.
DLPFC
- DLPFC is important for the maintenance, or “up-regulation,” of sensory stimuli.
VENTROLATERAL PFC
In addition, right VLPFC activity was negatively correlated with dorsal ACC activity, suggesting that the VLPFC was regulating neural activity related to the social distress of being excluded. These findings converge with the idea that right VLPFC mediates cognitive evaluation that mitigates neural activity associated with the affective component of pain.
Ventrolateral prefrontal cortex
The ventrolateral prefrontal cortex mediates some of the cognitive responses to negative emotions. In particular, depression seems to activate the left ventrolateral prefrontal cortex, which in turn enables individuals to maintain their focus on a specific and consequential problem, minimizing distractions. Anxiety appears to activate the right ventrolateral prefrontal cortex, which enhances the vigilance of individuals to anticipated hazards.
The ventrolateral prefrontal cortex is sometimes called the inferior frontal cortex. This structure corresponds to Brodmann's Areas 44, 45, and 47. Roles of the ventrolateral prefrontal cortex and dorsolateral prefrontal cortex
The difference between the ventrolateral prefrontal cortex and dorsolateral prefrontal cortex roughly aligns to the disparity between the ventral and dorsal pathways of the cortex, according to O'Reilly (2010). The ventral pathway, which is significantly underpinned by the temporal cortex, attempts to characterize the features and attributes of the stimuli in the environment, sometimes called the what system. The dorsal pathways, which is primarily underpinned by the parietal cortex, attempts to characterize the spatial relationships between stimuli and to ascertain which responses should be executed, called the how system.
The ventral pathways primarily terminate in the ventrolateral prefrontal cortex instead of the dorsolateral prefrontal cortex. Presumably, which features or attributes of the environment should be processed depends on the goals of individuals. The ventrolateral prefrontal cortex represents these goals and thus affects which features or attributes are extracted. That is, the ventrolateral prefrontal cortex biases or controls the ventral pathways.
The dorsal pathways primarily terminate in the dorsolateral prefrontal cortex instead of the ventrolateral prefrontal cortex. The dorsolateral prefrontal cortex represents complex relationships that can be applied, such as mathematical rules or other algorithms, to convert stimuli into responses. The dorsolateral prefrontal cortex, for example, may be able to retain a rule that was imposed a few minutes ago onto the dorsal pathway to ensure responses apply to these constraints.
O'Reilly (2010) accumulated considerable evidence to support this contention. For example, in one study, when individuals needed to differentiate stimuli that differ only on subtle semantic properties, demanding the what system, the ventrolateral prefrontal cortex was activated significantly. In contrast, when individuals needed to apply complex rules to decide which responses to execute, but the stimuli could be readily differentiated, the dorsolateral prefrontal cortex was activated significantly (Nagel et al., 2008).
Organization of the ventrolateral prefrontal cortex
Many studies indicate that broad or abstract concepts, such as “mammal”, are represented towards the front or rostral regions of the prefrontal cortex. In contrast, more specific or tangible concepts, such as “dog”, are represented caudally. According to O'Reilly (2010), this distinction between abstract and concrete representations probably pertains to the ventrolateral prefrontal cortex. For example, Brodmann area 47 may represent more abstract information than Brodmann area 44.
MEDIAL PFC
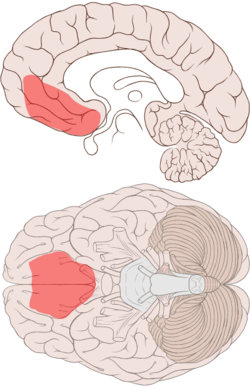
VENTROMEDIAL PFC
- implicated in the processing of risk, fear, and in decision making.
- Brodmann area 10.[2] However, not all sources agree on the boundaries of the area.
- Note that different researchers use the term 'Ventromedial prefrontal cortex' differently. Sometimes, the term is saved for the area above the medial orbitofrontal cortex, while at other times, 'ventromedial prefrontal cortex' is used to describe a broad area in the lower (ventral) central (medial) region of the prefrontal cortex, of which the medial orbitofrontal cortex constitutes the lower-most part. This latter, broader area corresponds to the area damaged in patients with decision-making impairments investigated
Function
- areas of the ventromedial cortex superior to the orbitofrontal cortex are much less associated with social functions and more with pure emotion regulation
- being associated with a decrease in cortisol levels
- Left lateral and medial orbitofrontal cortex areas were also measured to be highly active during guessing tasks. An increase in probabilistic scenario complexity was associated with orbitofrontal cortex activity level increase, therefore suggesting the special role that the ventromedial prefrontal cortex plays in decision making containing uncertainty. A study also indicated that patients with lesions in the ventromedial prefrontal cortex tend to have difficulties reacting to future consequences.
- The right half of the ventromedial prefrontal cortex was associated with regulating the interaction of cognition and affect in the production of empathic responses. Hedonic (pleasure) responses were also associations to orbitofrontal cortex activity level by Morten Kringelbach. This finding contributes findings suggesting ventromedial prefrontal cortex being associated with preference judgement, possibly assigning the ventromedial prefrontal cortex a key role in constructing one's self. Studies with PTSD also supported the idea that the ventromedial prefrontal cortex is an important component for reactivating past emotional associations and events, therefore essentially mediating pathogenesis of PTSD. Treatments geared to the inhibition of the ventromedial prefrontal cortex were therefore suggested for PTSD. The right half of the ventrolateral prefrontal cortex, being active during emotion regulation, was activated when participants were offered an unfair offer in a scenario. Specific deficits in reversal learning and decision-making have led to the hypothesis that the ventromedial prefrontal cortex is a major locus of dysfunction in the mild stages of the behavioural variant of frontotemporal dementia.
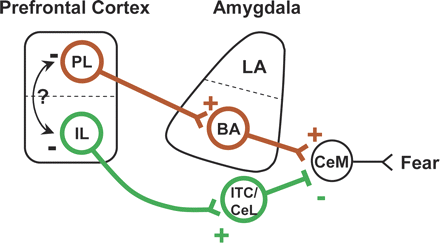
- vmPFC is homologous to rodent IL, whereas the dorsal regions of dACC are homologous to rodent PL
ANTERIOR PREFRONTAL CORTEX
http://www.pc.rhul.ac.uk/staff/n.ramnani/ramnani_owen.pdf
- 10p
- The results indicate a specific role for this region in integrating the outcomes of two or more separate cognitive
operations in the pursuit of a higher behavioural goal
The Truth about Lying: Inhibition of the Anterior Prefrontal Cortex Improves Deceptive Behavior http://cercor.oxfordjournals.org/content/20/1/205.long
Abstract Recent neuroimaging studies have indicated a predominant role of the anterior prefrontal cortex (aPFC) in deception and moral cognition, yet the functional contribution of the aPFC to deceptive behavior remains unknown. We hypothesized that modulating the excitability of the aPFC by transcranial direct current stimulation (tDCS) could reveal its functional contribution in generating deceitful responses. Forty-four healthy volunteers participated in a thief role-play in which they were supposed to steal money and then to attend an interrogation with the Guilty Knowledge Test. During the interrogation, participants received cathodal, anodal, or sham tDCS. Remarkably, inhibition of the aPFC by cathodal tDCS did not lead to an impairment of deceptive behavior but rather to a significant improvement. This effect manifested in faster reaction times in telling lies, but not in telling the truth, a decrease in sympathetic skin-conductance response and feelings of guilt while deceiving the interrogator and a significantly higher lying quotient reflecting skillful lying. Increasing the excitability of the aPFC by anodal tDCS did not affect deceptive behavior, confirming the specificity of the stimulation polarity. These findings give causal support to recent correlative data obtained by functional magnetic resonance imaging studies indicating a pivotal role of the aPFC in deception.
- anterior prefrontal cortices (aPFCs; BA 9/10) were engaged during general deception, but that the right aPFC was more involved in lies that were well rehearsed and were part of a coherent story than in spontaneous, noncoherent lies, whereas the ACC was more active during spontaneous generation of nonmemorized lies.
- The main effect of generating untruthful responses revealed increased brain activity of the left dorsolateral prefrontal cortex (DLPFC; BA 8) and the right aPFC, whereas the left ventromedial PFC (BA 11) and Amygdala were associated with the process of deceiving the interrogator. Further analysis revealed that only the right aPFC was associated with both factors of deception, indicating that this region has a pivotal role in telling lies.
- 4x6cm 1mA
PREFRONTAL CORTEX FUNCTION
http://www.columbia.edu/cu/psychology/tor/Papers/luria_vans_wager_sub.pdf
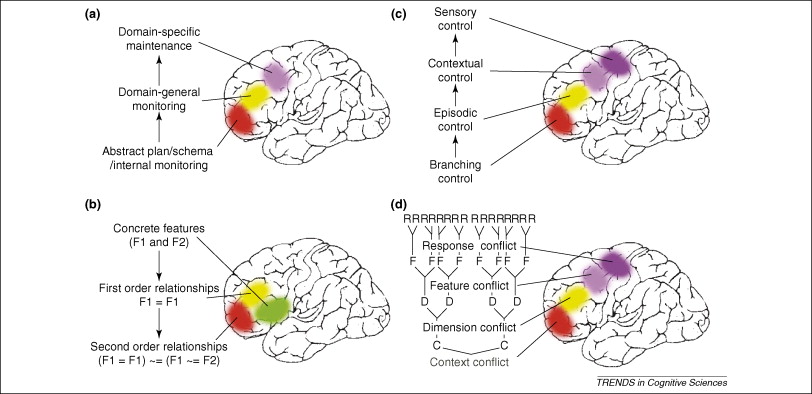
However, the overall picture of prefrontal cortex function presented here leads to a conceptualization of a cognitive processing hierarchy that proceeds along an anterior to posterior gradient, from a) representations of stimulus value in the OFC and rostral MPFC, to b) processing of internal goal and task-hierarchy representations in the ALPFC, c) top-down biasing of stimulus representation in posterior cortices by DLPFC, d) representation and updating of specific stimulus-response mapping rules in IFJ and lateral premotor cortex, e) the motivated planning of overt motor behavior in pre-SMA and cingulate motor areas, and f) the actual production of behavior in primary motor cortex. This notion of hierarchy is present in related forms in several current models of prefrontal function (e.g. Christoff & Keramatian, 2007; Koechlin et al., 2003). Of course, any kind of processing hierarchy in prefrontal cortex does not proceed in a truly linear fashion. One way to conceptualize cognitive control in the prefrontal cortex is as proceeding from the result of evaluations about the value of various stimuli or internal representations carried out in OFC. These valuations are then passed through the dorsal anterior insula to lateral prefrontal cortex, wherein DLPFC selects representations in posterior cortical regions that are task relevant and enhances their representation and/or inhibits the representation of task irrelevant representations. When information needs to be retrieved from LTM, the anterior portion of VLPFC is capable of initiating a controlled retrieval process, and if there are multiple competing active representations mid-VLPFC is recruited to select between them. The IFJ sets up S-R contingencies based on the current context, and directs the development of motor plans in supplementary motor cortex based on these contingencies. If additional processing on activated representations is required, for example the solution of intermediate processing stages or the completion of internally generated sub-goals, this is carried out by the RLPFC. Finally, if an incorrect response is generated and detected prior to its execution, the right IFG is brought online to inhibit the actual production of the response, and persistent energization of the entire system is maintained by the DMJ.
Resting-State Functional Connectivity of the Medial Superior Frontal Cortex

Short frontal lobe connections of the human brain

Sensory Pathways and Emotional Context for Action in Primate Prefrontal Cortex
Within the lateral prefrontal cortex, there are preferential targets of projections from visual, auditory, and somatosensory cortices associated with directing attention to relevant stimuli and monitoring responses for specific tasks. Return pathways from lateral prefrontal areas to sensory association cortices suggest a role in selecting relevant stimuli and suppressing distracters to accomplish specific tasks. Projections from sensory association cortices to orbitofrontal cortex are more global than to lateral prefrontal areas, especially for posterior orbitofrontal cortex (pOFC), which is connected with sensory association cortices representing each sensory modality and with structures associated with the internal, or emotional, environment. A specialized projection from pOFC to the intercalated masses of the amygdala is poised to flexibly affect autonomic responses in emotional arousal or return to homeostasis. The amygdala projects to the magnocellular mediodorsal thalamic nucleus, which projects most robustly to pOFC among prefrontal cortices, suggesting sequential processing for emotions. The specialized connections of pOFC distinguish it as a separate orbitofrontal region that may function as the primary sensor of information for emotions. Lateral prefrontal areas 46 and 9 and the pOFC send widespread projections to the inhibitory thalamic reticular nucleus, suggesting a role in gating sensory and motivationally salient signals and suppressing distracters at an early stage of processing. Intrinsic connections link prefrontal areas, enabling synthesis of sensory information and emotional context for selective attention and action, in processes that are disrupted in psychiatric disorders, including attention-deficit/hyperactivity disorder.
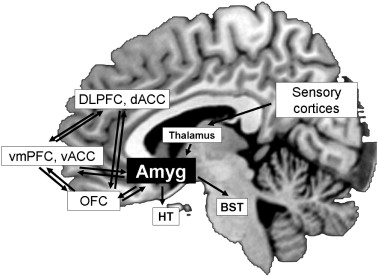
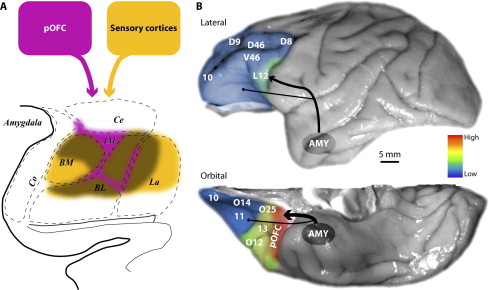 Connections between the prefrontal cortex and the amygdala. (A) Projections from posterior orbitofrontal cortex (pOFC) to the amygdala (magenta) target robustly the inhibitory intercalated masses in a unidirectional pathway. The pOFC also has bidirectional connections with the basal nuclei, where its connections overlap (brown) with sensory input (yellow) that reaches the amygdala from sensory association cortices. (B) Pseudo colored surface maps of the rhesus monkey brain show the strength of pathways from the amygdala that terminate in lateral (top) and orbital (bottom) prefrontal areas. The pOFC receives the strongest projections from the amygdala; the thickness of the arrows indicates pathway strength. AMY, amygdala; BL, basolateral nucleus; BM, basomedial nucleus; Ce, central nucleus; Co, cortical nuclei; IM, intercalated masses; La, lateral nucleus.
Connections between the prefrontal cortex and the amygdala. (A) Projections from posterior orbitofrontal cortex (pOFC) to the amygdala (magenta) target robustly the inhibitory intercalated masses in a unidirectional pathway. The pOFC also has bidirectional connections with the basal nuclei, where its connections overlap (brown) with sensory input (yellow) that reaches the amygdala from sensory association cortices. (B) Pseudo colored surface maps of the rhesus monkey brain show the strength of pathways from the amygdala that terminate in lateral (top) and orbital (bottom) prefrontal areas. The pOFC receives the strongest projections from the amygdala; the thickness of the arrows indicates pathway strength. AMY, amygdala; BL, basolateral nucleus; BM, basomedial nucleus; Ce, central nucleus; Co, cortical nuclei; IM, intercalated masses; La, lateral nucleus.
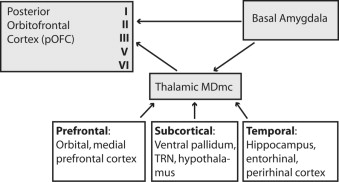 Schematic representation of direct projections from the amygdala to posterior orbitofrontal cortex, as well as indirect projections through the thalamic mediodorsal magnocellular (MDmc) nucleus, which is innervated by the amygdala. The terminations in the cortex show the predominance of direct amygdalar projections to the superficial layers and MDmc projections mostly to the middle cortical layers. The strong reciprocal projections from orbitofrontal cortex to MDmc and from posterior orbitofrontal cortex to the amygdala are not shown. TRN, thalamic reticular nucleus.
Schematic representation of direct projections from the amygdala to posterior orbitofrontal cortex, as well as indirect projections through the thalamic mediodorsal magnocellular (MDmc) nucleus, which is innervated by the amygdala. The terminations in the cortex show the predominance of direct amygdalar projections to the superficial layers and MDmc projections mostly to the middle cortical layers. The strong reciprocal projections from orbitofrontal cortex to MDmc and from posterior orbitofrontal cortex to the amygdala are not shown. TRN, thalamic reticular nucleus.
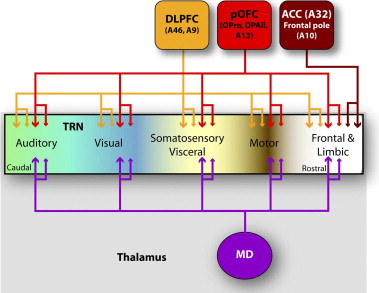 Prefrontal and mediodorsal thalamic projections to the thalamic reticular nucleus (TRN). Different TRN sectors are color-coded based on projections from cortical areas (frontal and limbic: white; motor: brown; somatosensory/visceral: yellow; visual: blue; auditory: green). Projections from all prefrontal cortices and the mediodorsal nucleus in the rhesus monkey are concentrated in the rostral (prefrontal) sector of TRN. However, mediodorsal nucleus, dorsolateral prefrontal (areas 46 and 9), and the posterior orbitofrontal cortex also extend projections to the central and posterior sectors of TRN, potentially influencing the flow of information from sensory and motor thalamic nuclei to the cortex. In addition, prefrontal
Prefrontal and mediodorsal thalamic projections to the thalamic reticular nucleus (TRN). Different TRN sectors are color-coded based on projections from cortical areas (frontal and limbic: white; motor: brown; somatosensory/visceral: yellow; visual: blue; auditory: green). Projections from all prefrontal cortices and the mediodorsal nucleus in the rhesus monkey are concentrated in the rostral (prefrontal) sector of TRN. However, mediodorsal nucleus, dorsolateral prefrontal (areas 46 and 9), and the posterior orbitofrontal cortex also extend projections to the central and posterior sectors of TRN, potentially influencing the flow of information from sensory and motor thalamic nuclei to the cortex. In addition, prefrontal
 Summary of amygdaloid outputs. Left: diagram of amygdaloid circuits involving the striatum pallidum medial thalamus and prefrontal cortex and output to the hypothalamus and brainstem. Right: diagram of areas of the cerebral cortex that receive axonal projections from the amygdala. The dark, medium, and lightly shaded areas represent high, medium, and low density of amygdaloid fibers. Modified from Amaral et al, 1992.
Summary of amygdaloid outputs. Left: diagram of amygdaloid circuits involving the striatum pallidum medial thalamus and prefrontal cortex and output to the hypothalamus and brainstem. Right: diagram of areas of the cerebral cortex that receive axonal projections from the amygdala. The dark, medium, and lightly shaded areas represent high, medium, and low density of amygdaloid fibers. Modified from Amaral et al, 1992.
 Anatomical circuits involving the medial prefrontal network (medial prefrontal network) and amygdala. Glutamatergic, presumed excitatory projections are shown in green, GABAergic projections are shown in orange, and modulatory projections in blue. In the model proposed here, dysfunction in the amygdala and/or the medial prefrontal network results in dysregulation of transmission throughout an extended brain circuit that stretches from the cortex to the brainstem, yielding the emotional, cognitive, endocrine, autonomic, and neurochemical manifestations of depression. Intra-amygdaloid connections link the basal and lateral amygdaloid nuclei to the central and medial nuclei of the amygdala and the bed nucleus of the stria terminalis (BNST). Parallel and convergent efferent projections from the amygdala and the medial prefrontal network to the hypothalamus, periaqueductal gray (PAG), nucleus basalis, locus ceruleus, dorsal raphe, and medullary vagal nuclei organize neuroendocrine, autonomic, neurotransmitter and behavioral responses to stressors and emotional stimuli (Davis and Shi, 1999, LeDoux, 2003). In addition, the amygdala and medial prefrontal network interact with the same cortico-striatal-pallidal-thalamic loop, through prominent connections both with the accumbens nucleus and medial caudate, and with the mediodorsal and paraventricular thalamic nuclei, which may function to control and limit responses to stress. Finally, the medial prefrontal network is a central node in the cortical 'default system' that appears to support self-referential functions such as mood. Other abbreviations: 5-HT—serotonin; ACh—acetylcholine; Cort.—corticosteroid; CRH—corticotrophin releasing hormone; Ctx—cortex; NorAdr—norepinephrine; PVN—paraventricular nucleus of the hypothalamus; PVZ—periventricular zone of hypothalamus; STGr—rostral superior temporal gyrus—VTA—ventral tegmental area.
Anatomical circuits involving the medial prefrontal network (medial prefrontal network) and amygdala. Glutamatergic, presumed excitatory projections are shown in green, GABAergic projections are shown in orange, and modulatory projections in blue. In the model proposed here, dysfunction in the amygdala and/or the medial prefrontal network results in dysregulation of transmission throughout an extended brain circuit that stretches from the cortex to the brainstem, yielding the emotional, cognitive, endocrine, autonomic, and neurochemical manifestations of depression. Intra-amygdaloid connections link the basal and lateral amygdaloid nuclei to the central and medial nuclei of the amygdala and the bed nucleus of the stria terminalis (BNST). Parallel and convergent efferent projections from the amygdala and the medial prefrontal network to the hypothalamus, periaqueductal gray (PAG), nucleus basalis, locus ceruleus, dorsal raphe, and medullary vagal nuclei organize neuroendocrine, autonomic, neurotransmitter and behavioral responses to stressors and emotional stimuli (Davis and Shi, 1999, LeDoux, 2003). In addition, the amygdala and medial prefrontal network interact with the same cortico-striatal-pallidal-thalamic loop, through prominent connections both with the accumbens nucleus and medial caudate, and with the mediodorsal and paraventricular thalamic nuclei, which may function to control and limit responses to stress. Finally, the medial prefrontal network is a central node in the cortical 'default system' that appears to support self-referential functions such as mood. Other abbreviations: 5-HT—serotonin; ACh—acetylcholine; Cort.—corticosteroid; CRH—corticotrophin releasing hormone; Ctx—cortex; NorAdr—norepinephrine; PVN—paraventricular nucleus of the hypothalamus; PVZ—periventricular zone of hypothalamus; STGr—rostral superior temporal gyrus—VTA—ventral tegmental area.
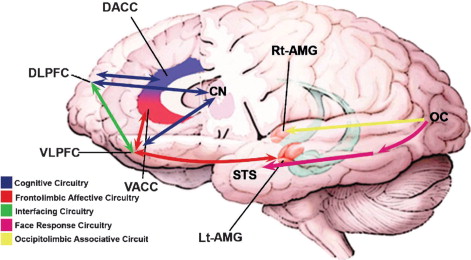
Functional brain circuits in pediatric bipolar disorder: cognitive circuitry shows the key link between the DLPFC and the caudate involved in attentional control and the connection between the VLPFC and the caudate/ventral striatum implicated in response inhibition and attention. Frontolimbic affective circuitry spanning from the VLPFC to the amygdala participates in top-down regulation of emotion. Interfacing circuitry illustrates the functional connectivity between cognitive regions (DLPFC and dorsal anterior cingulate cortex) and affective regions (VLPFC and VACC/pregenual and subgenual anterior cingulate cortex) at the level of the prefrontal cortex and the intermediary anterior cingulate cortex, working in concert in people without disorder. Face response circuitry connects the visual or occipital cortex with fusiform gyrus (not shown here) and the STS. The occipitolimbic associative circuitry connects the occipital cortex to the right amygdala, involved in fast or automatic processing of emotional/facial stimuli. DLPFC = dorsolateral prefrontal cortex; VACC = ventral anterior cingulate cortex; VLPFC = ventrolateral prefrontal cortex; STS = superior temporal sulcus.
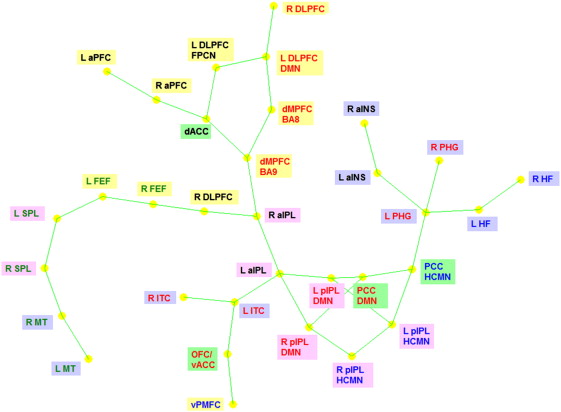 instantaneous correlation between the ROIs is shown as green lines. The distance between the nodes indicates the strength of their connection. The correspondence between ROI font colors and the RSNs is as follows. Red: default mode network (DMN), green: dorsal attention network (DAN), blue: hippocampal–cortical memory network (HCMN) and black: fronto-parietal control network (FPCN). The correspondence between ROI background colors and their anatomical location is as follows. Yellow: frontal cortex, pink: parietal cortex, blue: temporal cortex and green: cingulate cortex.
instantaneous correlation between the ROIs is shown as green lines. The distance between the nodes indicates the strength of their connection. The correspondence between ROI font colors and the RSNs is as follows. Red: default mode network (DMN), green: dorsal attention network (DAN), blue: hippocampal–cortical memory network (HCMN) and black: fronto-parietal control network (FPCN). The correspondence between ROI background colors and their anatomical location is as follows. Yellow: frontal cortex, pink: parietal cortex, blue: temporal cortex and green: cingulate cortex.
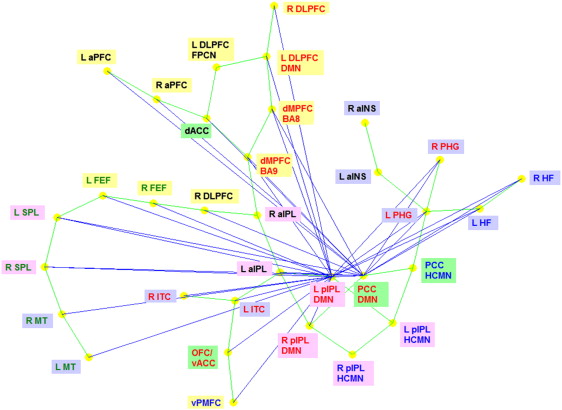 The reciprocal causal connections are shown as blue lines and the instantaneous correlation as green lines. The causal network formed by the blue lines represents the only strongly connected component in the entire network where in every ROI is mutually accessible causally by every other ROI.
The reciprocal causal connections are shown as blue lines and the instantaneous correlation as green lines. The causal network formed by the blue lines represents the only strongly connected component in the entire network where in every ROI is mutually accessible causally by every other ROI.
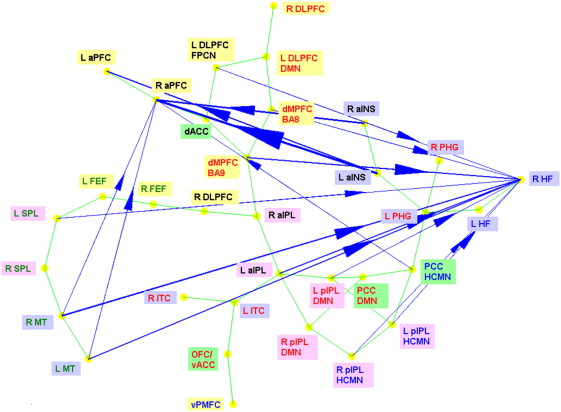 paths with unidirectional influences
paths with unidirectional influences
 Neural circuits of the social brain. Social perception in primates is largely visual [71], with faces having an important role in social interactions. Processing of visual social information occurs via specialized neural mechanisms, interacting with systems in humans that are concerned with the ability to experience empathy and to ‘mentalize’. This figure summarizes key aspects of neural connectivity in the social brain, in which amygdala (AMG) connectivity has a central role [72]. Abbreviations: ACC, anterior cingulate cortex; AMG, amygdala; FG, fusiform gyrus; Hipp, hippocampus; IFG, inferior frontal gyrus; IOG, inferior occipital gyrus; Ins, insula; MP, medial pulvinar nucleus of the thalamus; MPFC, mesial prefrontal cortex; N.S., nervous system; OFC, orbitofrontal cortex; PAG, periaquaductal gray; Pars Oper, pars opercularis; Pars Orb, pars orbitalis; Pars Tri, pars triangularis; PhippG, parahippocampal gyrus; pSTS, posterior superior temporal sulcus; Rostral IPL,rostral inferior parietal lobule; SC, superior colliculus; TPJ, temporoparietal junction; VPMC, ventral premotor cortex.
Neural circuits of the social brain. Social perception in primates is largely visual [71], with faces having an important role in social interactions. Processing of visual social information occurs via specialized neural mechanisms, interacting with systems in humans that are concerned with the ability to experience empathy and to ‘mentalize’. This figure summarizes key aspects of neural connectivity in the social brain, in which amygdala (AMG) connectivity has a central role [72]. Abbreviations: ACC, anterior cingulate cortex; AMG, amygdala; FG, fusiform gyrus; Hipp, hippocampus; IFG, inferior frontal gyrus; IOG, inferior occipital gyrus; Ins, insula; MP, medial pulvinar nucleus of the thalamus; MPFC, mesial prefrontal cortex; N.S., nervous system; OFC, orbitofrontal cortex; PAG, periaquaductal gray; Pars Oper, pars opercularis; Pars Orb, pars orbitalis; Pars Tri, pars triangularis; PhippG, parahippocampal gyrus; pSTS, posterior superior temporal sulcus; Rostral IPL,rostral inferior parietal lobule; SC, superior colliculus; TPJ, temporoparietal junction; VPMC, ventral premotor cortex.
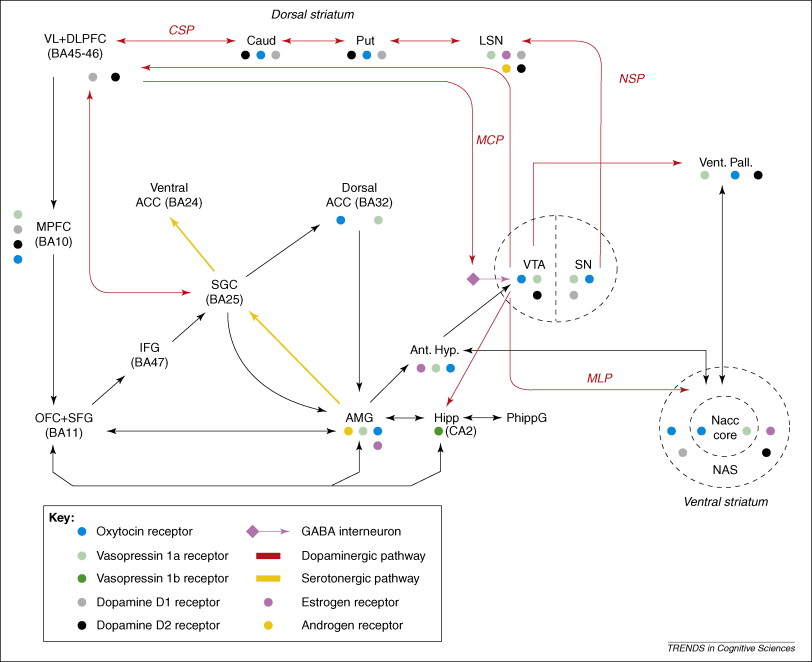 dopaminergic-neuropeptide interactions in the social brain. This figure is a simplified account of neural circuits that are believed to contribute to the regulation of the social brain. Only a subset of interconnections between brain regions involved in processing emotions and social perceptions is shown. There are also noradrenergic and serotonergic outputs (from the locus coeruleus and the dorsal raphe, respectively), which innervate most of the regions illustrated. Glutamatergic pathways not shown include inputs from frontal cortical regions, the hippocampus and amygdala to the VTA and nucleus accumbens (NAcc) [73]. Circuits potentially responsive to social reward encompass the corticostriatal pathway (CSP), the nigrostriatal pathway (NSP), the mesocortical pathway (MCP) and the mesolimbic pathway (MLP). These dopaminergic circuits link the dorsal and ventral striatum to the prefrontal cortex. The ventral striatum is involved in the prediction of future probable rewards as a consequence of personal actions. By contrast, the dorsal striatum monitors the outcome of those actions, querying ‘was the reward achieved?’ to choose better actions in future [74]. The model is an oversimplification of the organization of neural systems that underpin the complex human interplay involved in social interactions [75]. Abbreviations: ACC, anterior cingulate cortex; AMG, amygdala; Ant. Hypl, anterior hypothalamus; BA, Brodmann area; Caud, caudate; CSP, corticostriatal pathway; Hipp, hippocampus; IFG, inferior frontal gyrus; LSN, lat
dopaminergic-neuropeptide interactions in the social brain. This figure is a simplified account of neural circuits that are believed to contribute to the regulation of the social brain. Only a subset of interconnections between brain regions involved in processing emotions and social perceptions is shown. There are also noradrenergic and serotonergic outputs (from the locus coeruleus and the dorsal raphe, respectively), which innervate most of the regions illustrated. Glutamatergic pathways not shown include inputs from frontal cortical regions, the hippocampus and amygdala to the VTA and nucleus accumbens (NAcc) [73]. Circuits potentially responsive to social reward encompass the corticostriatal pathway (CSP), the nigrostriatal pathway (NSP), the mesocortical pathway (MCP) and the mesolimbic pathway (MLP). These dopaminergic circuits link the dorsal and ventral striatum to the prefrontal cortex. The ventral striatum is involved in the prediction of future probable rewards as a consequence of personal actions. By contrast, the dorsal striatum monitors the outcome of those actions, querying ‘was the reward achieved?’ to choose better actions in future [74]. The model is an oversimplification of the organization of neural systems that underpin the complex human interplay involved in social interactions [75]. Abbreviations: ACC, anterior cingulate cortex; AMG, amygdala; Ant. Hypl, anterior hypothalamus; BA, Brodmann area; Caud, caudate; CSP, corticostriatal pathway; Hipp, hippocampus; IFG, inferior frontal gyrus; LSN, lat
 Neural model of emotion regulation illustrating neural systems implicated in voluntary and automatic subprocesses of emotion regulation. (a) Feedforward pathway: medial prefrontal cortical system, including the OFC, subgenual ACG, rostral ACG, hippocampus and parahippocampus and MdPFC. (b) Feedback pathway: lateral prefrontal cortical system, including DLPFC and VLPFC. DLPFC, dorsolateral prefrontal cortex; MdPFC, dorsomedial prefrontal cortex; ACG, anterior cingulate gyrus; VLPFC, ventrolateral prefrontal cortex; OFC, orbital frontal cortex; hipp/parahip, hippocampus-parahippocampus region.
These abnormalities, which appear to be predominantly within the left-sided ventromedial prefrontal cortical regions implicated in automatic emotion regulation, may underlie the mood instability of adult bipolar disorder (BD). For example, functional neuroimaging studies have demonstrated greater subcortical limbic activity (including amygdala, ventral striatum and hippocampus) to emotional stimuli in adult BD relative to healthy individuals during mania,136, 147 depression137 and when euthymic.139, 140, 161, 162 Studies employing automatic attentional control paradigms show reduced activity predominantly in left-sided ventromedial PFC in BD relative to healthy adults.149, 150 Studies employing automatic emotion regulation paradigms also show reduced activity predominantly within left-sided ventromedial prefrontal cortical regions implicated in automatic emotion regulation, both during remission and mania in BD relative to healthy adults.157, 159 Structural neuroimaging findings show gray matter structural changes in left OFC, and abnormal integrity and number of white matter fibers connecting left OFC and subcortical limbic regions implicated in emotion processing, in adult BD.112, 120, 126, 131, 132, 133 There are more inconsistent findings regarding the roles of lateral and dorsal prefrontal cortical regions implicated in voluntary emotion regulation in adult BD. For example, findings from studies employing voluntary attentional control paradigms per se demonstrate patterns of reduced,143, 144 although also increased,145, 152 activity in different bilateral lateral and dorsal prefrontal cortical regions in BD vs healthy adults, whereas findings from studies employing voluntary emotion regulation paradigms indicate greater activity in BD than healthy adults bilaterally in these lateral and dorsal prefrontal cortical regions, together with greater activity in bilateral ventromedial prefrontal cortical regions implicated in automatic emotion regulation.140,155 The latter may mediate the voluntary emotion regulatory roles of the previous lateral and dorsal prefrontal cortical regions during voluntary emotion regulation.14 DLPFC, dorsolateral prefrontal cortex; MdPFC, dorsomedial prefrontal cortex; ACG, anterior cingulate gyrus; VLPFC, ventrolateral prefrontal cortex; OFC, orbital frontal cortex; hipp/parahip, hippocampus-parahippocampus region.
Neural model of emotion regulation illustrating neural systems implicated in voluntary and automatic subprocesses of emotion regulation. (a) Feedforward pathway: medial prefrontal cortical system, including the OFC, subgenual ACG, rostral ACG, hippocampus and parahippocampus and MdPFC. (b) Feedback pathway: lateral prefrontal cortical system, including DLPFC and VLPFC. DLPFC, dorsolateral prefrontal cortex; MdPFC, dorsomedial prefrontal cortex; ACG, anterior cingulate gyrus; VLPFC, ventrolateral prefrontal cortex; OFC, orbital frontal cortex; hipp/parahip, hippocampus-parahippocampus region.
These abnormalities, which appear to be predominantly within the left-sided ventromedial prefrontal cortical regions implicated in automatic emotion regulation, may underlie the mood instability of adult bipolar disorder (BD). For example, functional neuroimaging studies have demonstrated greater subcortical limbic activity (including amygdala, ventral striatum and hippocampus) to emotional stimuli in adult BD relative to healthy individuals during mania,136, 147 depression137 and when euthymic.139, 140, 161, 162 Studies employing automatic attentional control paradigms show reduced activity predominantly in left-sided ventromedial PFC in BD relative to healthy adults.149, 150 Studies employing automatic emotion regulation paradigms also show reduced activity predominantly within left-sided ventromedial prefrontal cortical regions implicated in automatic emotion regulation, both during remission and mania in BD relative to healthy adults.157, 159 Structural neuroimaging findings show gray matter structural changes in left OFC, and abnormal integrity and number of white matter fibers connecting left OFC and subcortical limbic regions implicated in emotion processing, in adult BD.112, 120, 126, 131, 132, 133 There are more inconsistent findings regarding the roles of lateral and dorsal prefrontal cortical regions implicated in voluntary emotion regulation in adult BD. For example, findings from studies employing voluntary attentional control paradigms per se demonstrate patterns of reduced,143, 144 although also increased,145, 152 activity in different bilateral lateral and dorsal prefrontal cortical regions in BD vs healthy adults, whereas findings from studies employing voluntary emotion regulation paradigms indicate greater activity in BD than healthy adults bilaterally in these lateral and dorsal prefrontal cortical regions, together with greater activity in bilateral ventromedial prefrontal cortical regions implicated in automatic emotion regulation.140,155 The latter may mediate the voluntary emotion regulatory roles of the previous lateral and dorsal prefrontal cortical regions during voluntary emotion regulation.14 DLPFC, dorsolateral prefrontal cortex; MdPFC, dorsomedial prefrontal cortex; ACG, anterior cingulate gyrus; VLPFC, ventrolateral prefrontal cortex; OFC, orbital frontal cortex; hipp/parahip, hippocampus-parahippocampus region.
The Neurocircuitry of Emotion Regulation http://www.nature.com/npp/journal/v35/n1/full/npp2009121a.html
In this review, we examine the functional architecture underlying the regulation of fear, focusing on four different types of regulatory processes: extinction, cognitive emotion regulation, active coping, and reconsolidation. During extinction, fear is diminished through learning that a previously threatening stimulus no longer signals danger. Cognitive emotion regulation involves using various mental strategies to modify a fear response. In active coping, fear is regulated through the performance of behaviors that reduce exposure to a fear-evoking stimulus. Finally, a fear memory can be disrupted after it is recalled through pharmacological or behavioral manipulations that block its reconsolidation. Our understanding of the neurocircuitry underlying the control of fear stems from research across species clarifying the mechanisms by which we learn and modify emotional associations, as well as studies exploring forms of cognitive emotion regulation that are uniquely human. In each section, we first review what is known about the neurocircuitry of the regulatory method from the non-human animal literature, followed by a review of the evidence available from human studies. We also briefly highlight the relevance of each regulatory method to the treatment of fear-related anxiety disorders.
CS-conditioned stimulus US-unconditioned stimulus
EXTINCTION Extinction learning involves the formation of a novel stimulus-outcome association. The CS that earlier predicted danger now predicts safety. This new extinction memory does not erase or overwrite the memory for the original CS–US association. This is evidenced by the re-emergence of the CR in certain circumstances including a shift in context (renewal), unsignaled presentation of the US (reinstatement), or the mere passage of time (spontaneous recovery)
neural mechanisms of fear conditioning across species indicate that the amygdala has a critical function in the acquisition, storage, and expression of conditioned fear. The lateral nucleus (LA) of the amygdala is thought to encode the association between CS- and US-related sensory inputs. When the CS is present, the LA excites the central nucleus (CE), which controls passive forms of expression of the CR through descending projections to the brainstem and hypothalamus. The LA also has indirect projections to the CE, through the basal nucleus (B) and the intercalated (ITC) cell masses, clusters of inhibitory GABAergic neurons. The B itself also projects directly to the ITC. These pathways provide multiple potential circuits for gating fear expression. Knowledge of the fear conditioning circuitry has allowed researchers to investigate functional changes that occur during extinction.
This body of research suggests that interaction between the amygdala, the ventromedial prefrontal cortex (vmPFC), and the hippocampus supports the acquisition, storage, retrieval, and contextual modulation of fear extinction
Although the amygdala seems to be critical for the acquisition of extinction learning, convergent evidence suggests that the vmPFC is necessary for the retention and recall of extinction. In line with the well-documented observation in human beings and primates that damage to the PFC leads to perseverative behavior observed that rats with vmPFC lesions required many more unreinforced presentations of the CS to extinguish conditioned responding. A subsequent study pointed to the infralimbic (IL) region of the vmPFC as a potential site of extinction consolidation, reporting that pre-training IL lesions left within-session acquisition of extinction intact, but impaired extinction retrieval on the following day
administration of an NMDA antagonist within the vmPFC all impair retrieval of extinction suggest that the plasticity in this region supports extinction consolidation
After extinction, contextual information has a critical function in determining whether the original fear memory or the new extinction memory should control fear expression . Evidence suggests that hippocampal projections to the vmPFC and the amygdala mediate the context-dependent expression of extinction
Furthermore, inactivation of the hippocampus before extinction learning impairs extinction recall on the subsequent day, suggesting that the hippocampus regulates fear expression both outside and within the extinction context. One suggestion is that the hippocampus controls the context-specific retrieval of extinction through projections to the vmPFC
studies have also observed increased vmPFC activation during extinction retrieval
hypocampus may mediate context-dependent extinction recall through connections with the vmPFC
Finally, a recent study reported that mice lacking the serotonin transporter gene show marked deficits in extinction retention (Wellman et al, 2007). As human beings with the low-expressing short allele variant of this gene exhibit decreased functional connectivity between the vmPFC and amygdala (Pezawas et al, 2005), this suggests a possible genetic basis for individual differences in extinction learning, as well as a potential risk factor for the development of anxiety disorders.
COGNITIVE REGULATION STRATEGIES Many different cognitive strategies can be used to regulate emotion, including reappraisal, selective attention, and suppression
cognitive regulation techniques often require the active engagement of the participants
reappraisal of negative scenes, as opposed to just attending to them, resulted in increased activation of both dorsolateral PFC (DLPFC) and ventrolateral PFC (VLPFC) regions along with dorsal anterior cingulate, and decreased activation of a region of the orbitofrontal cortex and the amygdala
It was proposed that underlying the reappraisal of negative effect, the engagement of the DLPFC may be linked to executive control processes required in the online manipulation of the interpretation of scenes, and the decrease of amygdala activation may reflect the cognitive control of subcortical mechanisms linked to the representation of negative emotional value
studies consistently report decreased amygdala activation and increased activation of the DLPFC and/or VLPFC, along with some involvement of medial PFC (mPFC) regions.
In this model, the DLPFC is involved in the effortful manipulation or interpretation of the stimulus and the VLPFC may have a function in the selection of emotion interpretation . The changes observed in the amygdala are the result of the top down modulation of the emotional meaning of the stimulus. One important aspect of this model is that the DLPFC does not project directly to the amygdala
Instead its influence on the amygdala is likely mediated by ventral and mPFC regions that have stronger connections with the amygdala . Although this model of emotion regulation is somewhat speculative
ACTIVE COPING parts of amygdala
RECONSOLIDATION During this consolidation period, it is possible to disrupt the formation of the memory, but once this time window has passed, the memory may be modified or inhibited, but not eliminated. However, recent studies support an alternative view of memory in which every time a memory is retrieved, the underlying memory trace is once again labile and fragile, requiring another consolidation period called reconsolidation. This reconsolidation period allows another opportunity to disrupt the memory.
As outlined above, extinction training usually results in two competing memory traces, a CS–US trace that competes for expression with a CS–noUS trace. As both traces exist, fear can return with standard extinction training, as different circumstances favor the expression of one trace over another (Bouton, 2004). However, the study by Monfils et al (2009) suggests that extinction training during the reconsolidation window results in an alternative memory representation. When extinction learning occurs during the time period in which the original CS–US trace is labile, this original trace may be significantly altered to incorporate the CS–noUS learning before re-storage. The result is either an alternative or combined memory trace representing the significance of the CS. Importantly, this new memory trace does not support the return of fear.
 Model for the neurocircuitry of fear regulation in humans through extinction, cognitive regulation, active coping, and reconsolidation. A network of structures including the amygdala, hippocampus, vmPFC, dlPFC, and the striatum are involved in the regulation of conditioned fear expression. The lateral nucleus (LA) of the amygdala receives afferent sensory input and is the site of CS–US plasticity during fear conditioning. The LA projects to the central nucleus (CE), which has outputs to regions that control the expression of the CR. Projections from the hippocampus to the basal nucleus (B) of the amygdala process contextual information during conditioning, and may gate fear expression through the CE. During extinction learning and consolidation, inhibitory connections between the vmPFC and the intercalated (ITC) cell masses are established. During extinction recall, these connections inhibit fear expression through projections to the CE. Inhibitory connections between the vmPFC and the LA may also regulate fear expression during extinction recall through the CE. Contextual modulation of extinction expression is mediated by projections from the hippocampus to the vmPFC and/or LA. During cognitive regulation, the dorsolateral prefrontal cortex (dlPFC) regulates fear expression through projections to the vmPFC, which in turn inhibits amygdala activity. During active coping, information from the LA is routed not to the CE, which drives fear expression, but to the B, which in turn projects to the striatum. The striatum is thought to reinforce instrumental action taken during escape-from-fear or avoidance learning. Reconsolidation diminishes conditioned fear expression through alteration of the original CS–US association stored in the LA.
Model for the neurocircuitry of fear regulation in humans through extinction, cognitive regulation, active coping, and reconsolidation. A network of structures including the amygdala, hippocampus, vmPFC, dlPFC, and the striatum are involved in the regulation of conditioned fear expression. The lateral nucleus (LA) of the amygdala receives afferent sensory input and is the site of CS–US plasticity during fear conditioning. The LA projects to the central nucleus (CE), which has outputs to regions that control the expression of the CR. Projections from the hippocampus to the basal nucleus (B) of the amygdala process contextual information during conditioning, and may gate fear expression through the CE. During extinction learning and consolidation, inhibitory connections between the vmPFC and the intercalated (ITC) cell masses are established. During extinction recall, these connections inhibit fear expression through projections to the CE. Inhibitory connections between the vmPFC and the LA may also regulate fear expression during extinction recall through the CE. Contextual modulation of extinction expression is mediated by projections from the hippocampus to the vmPFC and/or LA. During cognitive regulation, the dorsolateral prefrontal cortex (dlPFC) regulates fear expression through projections to the vmPFC, which in turn inhibits amygdala activity. During active coping, information from the LA is routed not to the CE, which drives fear expression, but to the B, which in turn projects to the striatum. The striatum is thought to reinforce instrumental action taken during escape-from-fear or avoidance learning. Reconsolidation diminishes conditioned fear expression through alteration of the original CS–US association stored in the LA.
We show that activity in right rostrolateral prefrontal cortex (rlPFC) satisfies three constraints for a role in metacognitive aspects of decision-making.
*These cognitive and motor systems, respectively, include cortical networks (prefronto-parietal and precentral regions) as well as subregions of the dorsal basal ganglia (caudate and putamen). Both systems appeared sensitive to incentive motivation: their activity increases when we work for higher rewards.
ventral prefrontal cortex and the ventral basal ganglia, has been implicated in encoding expected rewards